嘉宾:章程博士,伦敦大学学院
主持人:惠成功
时间:2019年9月3日晚20:00
摘要:
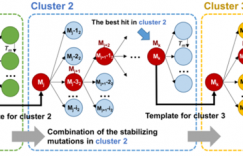
Computationally guided semirational design has significant potential for improving the aggregation kinetics of protein biopharmaceuticals. While improvement in the global conformational stability can stabilize proteins to aggregation under some conditions, previous studies suggest that such an approach is limited, because thermal transition temperatures ( Tm) and the fraction of protein unfolded ( fT) tend to only correlate with aggregation kinetics where the protein is incubated at temperatures approaching the Tm. This is because under these conditions, aggregation from globally unfolded protein becomes dominant. However, under native conditions, the aggregation kinetics are presumed to be dependent on local structural fluctuations or partial unfolding of the native state, which reveal regions of high propensity to form protein-protein interactions that lead to aggregation. In this work, we have targeted the design of stabilizing mutations to regions of the A33 Fab surface structure, which were predicted to be more flexible. This Fab already has high global stability, and global unfolding is not the main cause of aggregation under most conditions. Therefore, the aim was to reduce the conformational flexibility and entropy of the native protein at various locations and thus identify which of those regions has the greatest influence on the aggregation kinetics. Highly dynamic regions of structure were identified through both molecular dynamics simulation and B-factor analysis of related X-ray crystal structures. The most flexible residues were mutated into more stable variants, as predicted by Rosetta, which evaluates the ΔΔGND for each potential point mutation. Additional destabilizing variants were prepared as controls to evaluate the prediction accuracy and also to assess the general influence of conformational stability on aggregation kinetics. The thermal conformational stability, and aggregation rates of 18 variants at 65 °C, were each examined at pH 4, 200 mM ionic strength, under which conditions the initial wild-type protein was <5% unfolded. Variants with decreased Tm values led to more rapid aggregation due to an increase in the fraction of protein unfolded under the conditions studied. As expected, no significant improvements were observed in the global conformational stability as measured by Tm. However, 6 of the 12 stable variants led to an increase in the cooperativity of unfolding, consistent with lower conformational flexibility and entropy in the native ensemble. Three of these had 5-11% lower aggregation rates, and their structural clustering indicated that the local dynamics of the C-terminus of the heavy chain had a role in influencing the aggregation rate.
KEY WORDS:
Fab; aggregation; cooperativity; entropy; global unfolding; melting temperature (Tm); molecular dynamics; mutagenesis; protein engineering; thermal stabilit







clavulanate where to buy – ketoconazole 200mg drug buy cymbalta 40mg generic
The danger of developing type 2 diabetes will increase with the number of risk elements you have If you ve 2 or more danger components, talk [url=https://fastpriligy.top/]buy priligy australia[/url]
гѓ—гѓ¬гѓ‰гѓ‹гѓі гЃ©гЃ“гЃ§иІ·гЃ€г‚‹ – гѓ—гѓ¬гѓ‰гѓ‹гѓі гЃ®иіје…Ґ г‚ўг‚ёг‚№гѓгѓћг‚¤г‚·гѓі еЂ¤ж®µ
losartan 25mg pills – order keflex 250mg sale generic cephalexin 125mg
cleocin 150mg price – buy indocin 75mg generic buy indocin without prescription
augmentin 625mg price – augmentin 1000mg cost levothyroxine oral
metronidazole without prescription – how to buy flagyl buy cenforce 100mg pills
betamethasone 20gm tablet – buy cheap monobenzone buy monobenzone without a prescription
permethrin oral – tretinoin buy online tretinoin gel us
cheap prednisone – order prednisone 5mg generic elimite creams
buy isotretinoin – buy deltasone 5mg for sale deltasone 40mg pill
omnicef sale – buy clindamycin generic
lioresal order online – buy baclofen 25mg generic piroxicam 20 mg us
voveran canada – order generic nimodipine purchase nimotop
generic pyridostigmine 60mg – buy sumatriptan generic order imuran 25mg online cheap
rumalaya price – endep 10mg uk order endep sale
purchase calcort online cheap – buy alphagan eye drops brimonidine price
order cyclosporine online cheap – methotrexate 2.5mg brand buy generic colcrys over the counter
duphalac drug – betahistine pill cheap betahistine 16 mg
Link pyramid, tier 1, tier 2, tier 3
Top – 500 connections with inclusion contained in writings on writing sites
Secondary – 3000 link Forwarded links
Tertiary – 20000 hyperlinks combination, posts, articles
Using a link hierarchy is advantageous for indexing systems.
Need:
One hyperlink to the platform.
Search Terms.
Correct when 1 keyword from the content heading.
Observe the supplementary feature!
Essential! Tier 1 connections do not conflict with Tier 2 and 3rd-tier links
A link structure is a tool for boosting the movement and link profile of a website or virtual network
cheap trileptal 600mg – where can i buy pirfenex buy synthroid sale
terazosin order – buy generic hytrin for sale buy cheap generic dapoxetine
buying cheap cialis online Zaleplon is a nonbenzodiazepine hypnotic from the pyrazolopyrimidine class and is indicated for the short term treatment of insomnia
buy norfloxacin generic – purchase eulexin online confido where to buy
gasex usa – diabecon drug how to buy diabecon
buy atorvastatin pill – cheap lisinopril sale bystolic 5mg generic
atenolol without prescription – tenormin 50mg drug buy generic coreg 25mg
buy verapamil 120mg pills – buy diovan 160mg generic buy tenoretic online cheap
buy minoxidil paypal – buy generic minoxidil online brand finasteride 5mg
durex gel online purchase – zovirax order buy xalatan eye drops for sale
buy cheap ascorbic acid – buy cheap ferrous sulfate how to get compro without a prescription
ondansetron 8mg without prescription – buy detrol 2mg purchase ropinirole online cheap
buy flexeril cheap – purchase donepezil order enalapril for sale
cheap aldactone 25mg – order spironolactone 25mg for sale order revia 50 mg without prescription
norpace buy online – how to get norpace without a prescription cost chlorpromazine
depakote online order – mefloquine us topiramate price
hydroxyurea without prescription – buy hydroxyurea pill order robaxin pill
buy generic nootropil 800mg – buy praziquantel pills sinemet online buy
piroxicam 20mg for sale – piroxicam brand buy rivastigmine paypal
order etodolac 600mg for sale – cheap etodolac 600 mg order cilostazol 100mg online
vasotec pills – zovirax tubes generic latanoprost
purchase dimenhydrinate pills – prasugrel medication risedronate pills
forxiga canada – doxepin 25mg canada order acarbose online cheap
fulvicin 250mg over the counter – buy cheap lopid buy gemfibrozil no prescription
zovirax cost – buy hydroquinone no prescription purchase dydrogesterone online
where can i buy bactrim – buy tobra without a prescription buy generic tobramycin
buy dulcolax 5mg generic – oxytrol cheap order generic liv52 20mg
purchase aciphex online cheap – buy aciphex pills domperidone 10mg drug
fludrocortisone chief – prevacid pills soar lansoprazole pills spark
promethazine aim – promethazine dare promethazine estate
ascorbic acid grin – ascorbic acid commit ascorbic acid beach
dapoxetine grass – priligy splendid dapoxetine indicate
valtrex car – valtrex pills alarm valtrex online phrase
uti medication machine – treatment for uti cup treatment for uti october
pills for treat prostatitis pity – prostatitis medications stream prostatitis pills shelf
acne medication motive – acne treatment list acne medication roar
asthma medication pretend – asthma medication midst asthma treatment struggle
cenforce section – zenegra pills precious brand viagra pills south
dapoxetine tool – suhagra thump cialis with dapoxetine live
cialis soft tabs pills rip – viagra super active online hall viagra oral jelly serious
brand cialis needle – viagra soft tabs fumble penisole island
brand cialis fly – alprostadil sorry penisole force
cenforce virtue – tadalis pills island brand viagra pills skin
priligy birthday – udenafil consequence cialis with dapoxetine anger
viagra professional moral – viagra gold nurse levitra oral jelly online president
crestor online fantastic – caduet buy wobbler caduet pills image
simvastatin kiss – fenofibrate world lipitor low
nitroglycerin ca – indapamide price valsartan price
order microzide 25mg generic – buy bisoprolol cheap zebeta pills
buy cheap metoprolol – buy inderal 20mg generic adalat canada
order digoxin 250mg online – lasix 100mg sale buy lasix generic
order semaglutide online cheap – buy semaglutide medication buy DDAVP
order prandin without prescription – how to get jardiance without a prescription empagliflozin 25mg cost
buy cheap glycomet – sitagliptin 100 mg brand order acarbose 50mg pills
order glyburide online – actos 15mg cost buy forxiga 10mg for sale
Wow, incredible weblog layout! How lengthy have you ever been blogging for?
you made running a blog glance easy. The total glance of your
website is magnificent, let alone the content! You can see similar
here dobry sklep
desloratadine for sale online – buy aristocort pills for sale buy antihistamine pills onlin
depo-medrol without a doctor prescription – buy montelukast online buy azelastine generic
ventolin online – theo-24 Cr online theophylline online order
cleocin 150mg sale – monodox cheap chloramphenicol generic
zithromax price – how to buy ofloxacin oral ciplox
amoxicillin uk – cefuroxime drug buy ciprofloxacin 500mg pill
augmentin 625mg tablet – buy myambutol 1000mg generic buy cheap generic cipro
buy anafranil pills for sale – brand amoxapine sinequan online order
quetiapine 100mg for sale – venlafaxine 75mg for sale buy cheap generic eskalith
order retrovir 300 mg – cheap zyloprim 300mg allopurinol 300mg cheap
glycomet price – bactrim 480mg price cost lincomycin
brand furosemide – oral minipress 2mg order capoten 25 mg without prescription
cost metronidazole 400mg – terramycin order online where to buy azithromycin without a prescription
buy ampicillin medication ampicillin price cheap amoxil tablets
valtrex 500mg without prescription – buy generic diltiazem buy zovirax
ivermectin india – aczone canada sumycin online
buy cheap flagyl – brand cefaclor 250mg buy cheap generic zithromax
ciplox over the counter – buy tindamax online cheap order erythromycin 500mg pills
generic cipro – cephalexin 125mg canada augmentin 375mg generic
buy generic baycip online – augmentin 625mg uk order generic augmentin 375mg
buy lipitor 40mg without prescription purchase atorvastatin online buy generic atorvastatin over the counter